
 Home Home |
 For authors For authors |
 Submission status Submission status |
 Current Current
|
 Archive Archive
|
 Archive
(English) Archive
(English)
|
 Search Search |
Laser ablation into liquid (LAL) is used to produce nanoparticles (NPs). Ultra-short ablation (femto- picosecond fs/ps-LAL) and nanosecond ablation (ns-LAL) are available. During fs/ps-LAL, cavity nucleation occurs beneath the irradiated surface. Then the detachment of the spallation layer (SpL) takes place. In the fs/ps-LAL, nucleation, foaming, and disintegration of the SpL significantly affect the number and size distribution of the resulting NPs.
There is no subsurface nucleation during ns-LAL considered here. There is no SpL, no capillary decay of the SpL. Then the standard process of NPs formation consists of three links: (1) evaporation - (2) diffusion in the receiving substance (which is air or liquid; in our case, liquid/water, see Figure) - (3) condensation.
At absorbed fluences F~1 J/cm2, the gold-water contact boundary (cb) is a few nanoseconds above the critical point in the gold phase diagram – this is the supercritical time interval. The importance of this circumstance is great. At this time interval the capillary barrier disappears, which should be overcome by evaporation (surface tension is zero). Then, firstly, the diffusion flux is sharply intensified and, secondly, cooling of the evaporating melt due to large heat of evaporation disappears. Thus, link 1 in the 1-2-3 chain drops out. Link 1 drastically reduces the amount of LF, see figure.
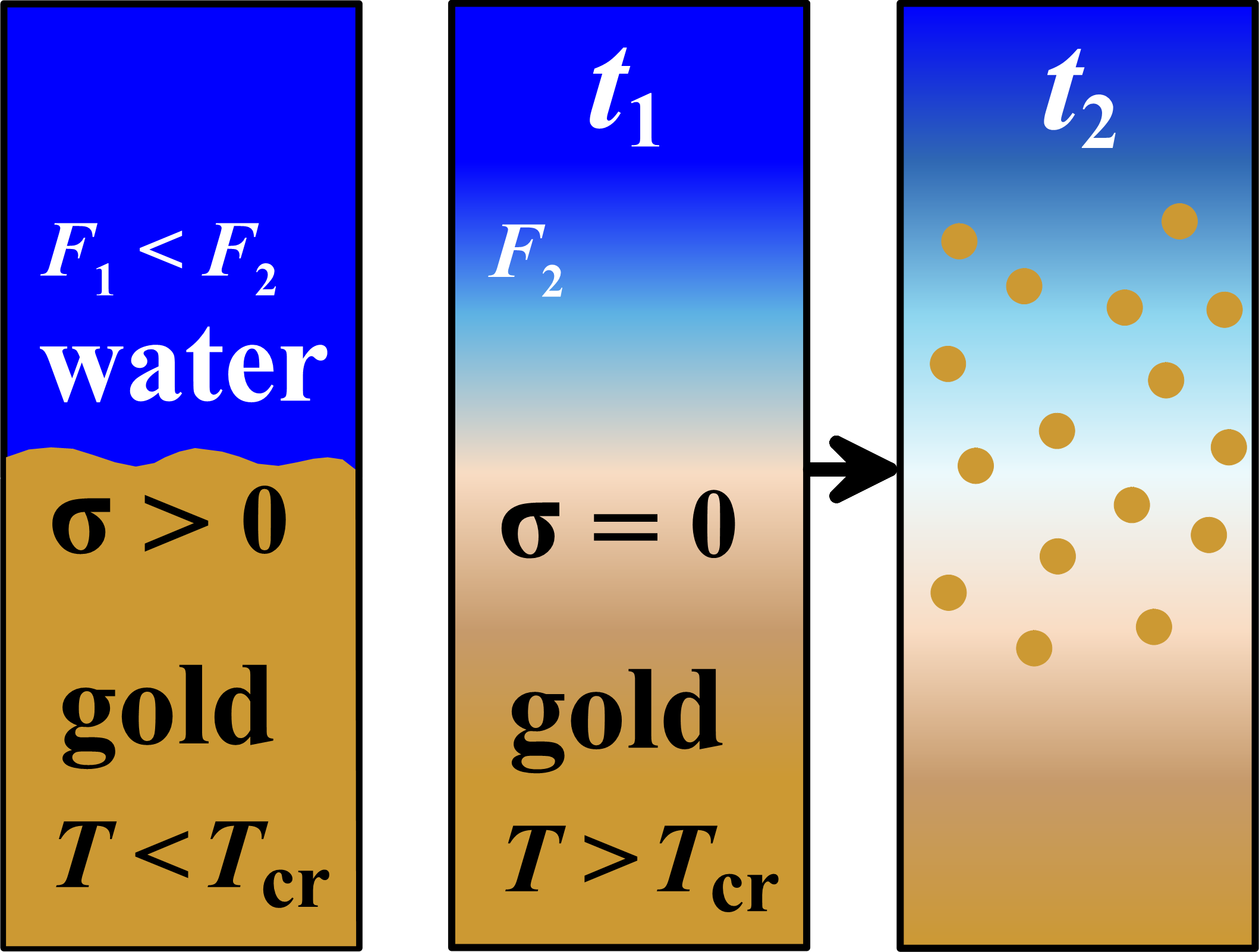
In the case of supercritical states, the entropy of gold Scb at contact boundary (cb) exceeds the critical entropy Scr. Gold of the [Scb-Scr] segment of the material profile comes under the binodal through the condensation curve (cc); except for the amount that diffused through point “cb” into the water. However, gold [Scb-Scr] does not form NPs!
Split the segment [Scb-Scr] into layers “S”: Scb > S > Scr. The layers “S” cross the condensation curve sequentially from lower entropy values to higher values. Consider two adjacent layers Scc > S of this sequence. Let the layer Scc cross the condensation curve “cc” at time t. Layer S must be in a two-phase state with saturated vapor pressure Psat(S,t). Pressure Psat(S,t) is less than the pressure Pcc = Psat(Scc,t). Therefore, the two-phase layer S collapses (shrinks) into a one-phase liquid. Accordingly, there is no NP contribution from the S layer.
N. A. Inogamov, V. V. Zhakhovsky, V. A. Khokhlov
JETP Letters 115, issue 1 (2022)