
 Home Home |
 For authors For authors |
 Submission status Submission status |
 Current Current
|
 Archive Archive
|
 Archive
(English) Archive
(English)
|
 Search Search |
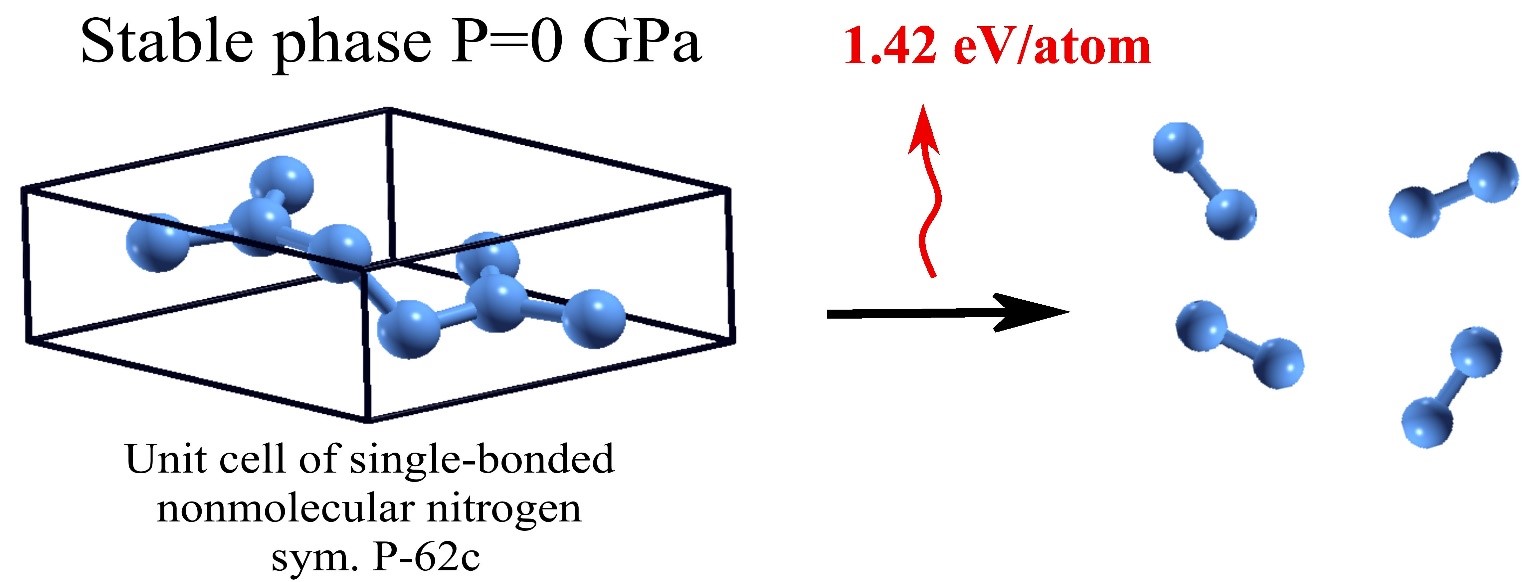
The problem of searching new high-energy-density materials (HEDM) is very actual from both applied and fundamental points of view. Choosing nitrogen as a promising element for creating HEDMs have several reasons. Under normal conditions, nitrogen exists in the form of diatomic N2 molecules with a triple covalent bond, which is one of the strongest covalent bonds in nature, its energy is 4.9 eV/atom. The energies of double and single bonds for nitrogen are 2.17 eV/atom and 0.83 eV/atom, respectively. Those for nitrogen the sum of three single bonds energies is much less than energy of triple bond; therefore, single-bonded nitrogen crystal structures will store energy. At the same time, the release of energy is an environmentally friendly process.
In this article, the existence of a metastable, single-bonded crystalline nitrogen phase with symmetry P-62c is predicted theoretically. This phase is a direct-gap semiconductor and can store the largest amount of energy among all nitrogen crystals predicted to date, which are stable at low pressures. This structure of non-molecular nitrogen has all the necessary attributes of dynamic (in terms of the phonon spectrum) and mechanical (in terms of elastic moduli) stability of a bulk medium at pressures less than 40 GPa, including zero pressure. In the entire pressure stability range phase P-62c is metastable. For its synthesis, it is necessary to search new methods, for example, synthesis through excited states.
K.S.Grishakov and N.N.Degtyarenko
JETP Letters 112, issue 10 (2020)