
| VOLUME 117 (2023) | ISSUE 5 | PAGE 385 |
|---|
|
Formal valence, charge distribution and chemical bond in a compound with a high oxidation state: KMnO4
V. I. Anisimov+×, A. R. Oganov*, M. A. Mazannikova+×, D. Y. Novoselov+×, Dm. M. Korotin+
+M. N. Mikheev Institute of Metal Physics of Ural Branch of Russian Academy of Sciences, 620108 Yekaterinburg, Russia ×Department of theoretical physics and applied mathematics, Ural Federal University, 620002 Yekaterinburg, Russia *Skolkovo Institute of Science and Technology, 121205 Moscow, Russia
Abstract
KMnO4 has unusual formal manganese valence state Mn+7 that seems puzzling: the energy of creating such ion (119 eV) is much greater than the energy of chemical bonds (up to 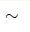 10 eV). We have used the Wannier
functions formalism to analyze the distribution of Mn-3d electrons and O-2p
electrons for empty electronic states in the MnO4- complex and
have found that, while formally one has d0 configuration for manganese
ion in this compound, in reality only about one-half of the hole density
described by these Wannier functions corresponding to this configuration
belongs to d-electrons, while the other half is spread over surrounding
oxygen atoms. That corresponds much more to Mn+2 state than to
Mn+7,
because calculated total number of d-electrons is equal to 5.25.
This analysis also has shown nearly perfect covalent type of chemical
bond within the MnO4- complex with negligible contribution of the ionic part. 10 eV). We have used the Wannier
functions formalism to analyze the distribution of Mn-3d electrons and O-2p
electrons for empty electronic states in the MnO4- complex and
have found that, while formally one has d0 configuration for manganese
ion in this compound, in reality only about one-half of the hole density
described by these Wannier functions corresponding to this configuration
belongs to d-electrons, while the other half is spread over surrounding
oxygen atoms. That corresponds much more to Mn+2 state than to
Mn+7,
because calculated total number of d-electrons is equal to 5.25.
This analysis also has shown nearly perfect covalent type of chemical
bond within the MnO4- complex with negligible contribution of the ionic part.
|
 Home
Home